
Background
Graft-versus-host disease (GVHD) is a serious, potentially fatal complication of allogeneic hematopoietic cell transplantation (HCT). Ruxolitinib (RUX), a Janus kinase (JAK) 1/JAK2 inhibitor, is in phase 3 development for patients (pts) with steroid-refractory chronic GVHD (SR cGVHD), and is approved for the treatment of steroid-refractory acute GVHD (SR aGVHD) in the United States. Outside of clinical trials, access to RUX is provided to pts with SR cGVHD through an expanded access program (EAP) sponsored by Incyte Corporation in the United States. The primary objective of this analysis was to report safety data from pts with SR cGVHD who received RUX in the Incyte-sponsored US EAP.
Study Design and Methods
Patients eligible to enroll in the open-label, multicenter US EAP from September 2017-May 2020 were ≥12 years of age, developed SR aGVHD or SR cGVHD after allogeneic HCT, and had an ECOG PS of 0-3. Pts with SR aGVHD and those with incomplete or missing data were excluded from the analysis. Based on clinical experience, the recommended starting dose of oral RUX for pts with cGVHD was 10 mg twice daily (BID). Doses could not be escalated above 10 mg BID, but dose reductions were permitted during treatment based on safety and laboratory assessments. The dose of RUX could be re-escalated if toxicity management thresholds were met or if pts experienced GVHD flares and had adequate hematologic parameters. Any GVHD treatments received before RUX initiation were recorded, and concurrent therapy with other cGVHD treatments was permitted. Serious adverse events (SAEs) were assessed according to National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 from the time of consent until 30 days after end of treatment. Pt characteristics, prior treatments, and RUX dosing were summarized using descriptive statistics. Overall survival (OS) was assessed using Kaplan-Meier methodology.
Results
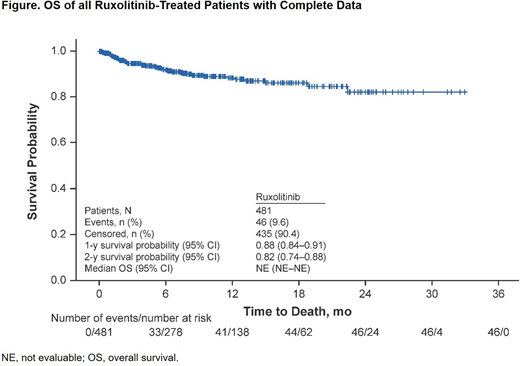
The analysis included 481 pts with SR cGVHD and complete data (data cutoff, 08 May 2020). Median (range) age was 60.0 (15-81) years; 51.8% of pts were male. At the time of RUX initiation, the organs involved included the skin (70.7%), eyes (59.3%), mouth (55.7%), joints (38.0%), lungs (34.9%), gastrointestinal tract (28.1%), liver (18.9%), and genitals (11.0%). Most pts (65.3%) received ≥2 prior treatments for GVHD, including systemic corticosteroids (54.9%), calcineurin inhibitors (25.6%), sirolimus (20.8%), mycophenolate mofetil (14.6%), ibrutinib (7.1%), and RUX (3.1%); 26.6% of pts received topical corticosteroids and 12.3% received other topical treatments. Most pts initially received RUX 5 mg BID (n=228 [47.4%]) or 10 mg BID (n=229 [47.6%]); 276 pts (57.4%) received RUX 10 mg BID as their last dose at the time of discontinuation or data cutoff. At data cutoff, 332 pts (69.0%) were still receiving RUX. Primary reasons for treatment discontinuations were death (7.1%), GVHD progression (5.4%), malignancy relapse (3.5%), and adverse events (2.5%). The median (range) duration of RUX treatment was 7.2 (0.03-33.0) months. SAEs, regardless of causality, were reported in 162 pts (33.7%). Median (range) time to first SAE from RUX initiation was 77.0 (1-693) days. The most common SAEs were sepsis (n=18 [3.7%]), pyrexia (n=9 [1.9%]), dyspnea (n=8 [1.7%]), respiratory failure (n=8 [1.7%]), acute kidney injury (n=7 [1.5%]), failure to thrive (n=7 [1.5%]), influenza (n=7 [1.5%]), diarrhea (n=6 [1.2%]), fall (n=6 [1.2%]), pulmonary embolism (n=6 [1.2%]), and upper respiratory tract infection (n=6 [1.2%]). One pt (0.2%) had a cytomegalovirus viremia SAE. There were few SAEs reported for cytopenias (febrile neutropenia, n=2 [0.4%]) or fungal infections (fungal pneumonia, n=1 [0.2%]); SAEs for neoplasms were reported for 8 pts (1.7%). There were no SAEs of thrombotic microangiopathy. Forty-six pts (9.6%) had fatal SAEs, most commonly attributed, at least in part, to infections (n=13 [28.3% of SAE-related fatalities]). Thirty-six pts (7.5%) had SAEs deemed related to RUX. The OS rates (95% CI) were 88% (84-91) at 1 year and 82% (74-88) at 2 years (Figure).
Conclusions
Patients with SR cGVHD in the RUX EAP program were heavily pretreated and primarily had organ involvement of the skin, eyes, and mouth. SAEs were reported in one-third of pts, including 7% of pts with RUX-related SAEs. Ten percent of pts had fatal SAEs, primarily due to infectious complications of cGVHD. No new or unexpected SAEs were reported.
Hari:Incyte Corporation: Consultancy; Takeda: Consultancy; BMS: Consultancy; Amgen: Consultancy; GSK: Consultancy; Janssen: Consultancy. Ali:Incyte Corporation: Consultancy. Chen:Takeda: Consultancy; Incyte Corporation: Consultancy; Equillium: Other: Data and Safety Monitoring Board Member; Actinium: Other: Data and Safety Monitoring Board Member; Magenta: Consultancy; Kiadis: Consultancy; AbbVie: Other: Data and Safety Monitoring Board Member. Fazal:Agios: Consultancy, Speakers Bureau; Jazz Pharma: Consultancy, Speakers Bureau; Jansen: Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Glaxosmith Kline: Consultancy, Speakers Bureau; Stemline: Consultancy, Speakers Bureau; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Karyopham: Speakers Bureau; Gilead/Kite: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Gregory:Bluebird: Research Funding; Celgene: Research Funding; BMS: Research Funding; CURIS: Research Funding; Celularity: Research Funding; Constellation: Research Funding; CRISP Therapeutics: Research Funding; Acetylon: Research Funding; AbbVie: Research Funding; Amgen: Research Funding; Incyte Corporation: Consultancy; Vivolux: Research Funding; Teva: Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Poseida: Research Funding; Novartis: Research Funding; Kesios: Research Funding; Lilly: Research Funding; Janssen: Research Funding; Glenmark: Research Funding; Genentech: Research Funding; EMD Sorono: Research Funding. Anand:AltruBio: Research Funding; CSL Behring: Research Funding; Incyte Corporation: Research Funding; Equillium: Research Funding; Kadmon: Research Funding. Naim:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Paranagama:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Bhatt:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Blithe:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. DiPersio:Magenta Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Ruxolitinib is a JAK1/JAK2 inhibitor approved by the FDA for the treatment of adults with steroid-refractory acute GVHD. Ruxolitinib is in phase 3 testing for the treatment of steroid-refractory chronic GVHD but is currently not approved for this indication.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal